No
About the Data
The Nebraska Influenza and other Respiratory Disease Surveillance System (NIRDSS) is a collaborative effort between DHHS and its many partners in the state including local health departments, public health and clinical laboratories, vital statistics offices, healthcare providers, clinics, and emergency departments. Respiratory virus surveillance data is analyzed across weekly time periods called MMWR weeks (see additional resources) and respiratory seasons (also may be referred to as an influenza or RSV season).
A respiratory season is defined as an entire year span beginning at MMWR week 40 (roughly the start of October) through week 39 (the week prior to 40 in the next calendar year). For example, the 2022-23 respiratory season began on week 40 of 2022 and ended week 39 of 2023.
Respiratory disease surveillance allows us to determine when we first start to see various respiratory disease activity, such as influenza, RSV, and COVID-19, each year. It provides an indicator of the progression of the respiratory disease season for these viruses, as well as prevalence of disease in the community, which assists healthcare providers in diagnosing patients with these various respiratory diseases. Surveillance additionally identifies what strains of influenza and COVID-19 are circulating in any given year, and thus determines whether the current vaccine protects against the circulating strain. This information also aids researchers in vaccine development to develop more effective vaccines by utilizing data from these circulating strains. By incorporating multiple data sources, we can communicate a more complete picture of respiratory disease activity to our communities.
The term 'ILI' or influenza-like illness is commonly used to track influenza activity in various surveillance systems, as seen throughout the influenza dashboard. ILI is defined as any patient with clinically diagnosed influenza or any patient with fever ≥ 100°F (≥ 37.8°C), oral or equivalent, AND cough and/or sore throat. The case definition no longer includes “without a known cause other than influenza".
See information about Morbidity and Mortality Weekly Report (MMWR) weeks.
See the 2024-2025 MMWR Week Calendar
For additional resources about respiratory diseases and surveillance data, please visit:
Geographic Regions
Within the laboratory sections of the dashboard, case rates per 100,000 population specific to each respiratory disease are displayed by Nebraska's 19 local health departments (LHDs). In the case rate calculations, US Census data was used to define the population estimates. The following map depicts these 19 LHDs:
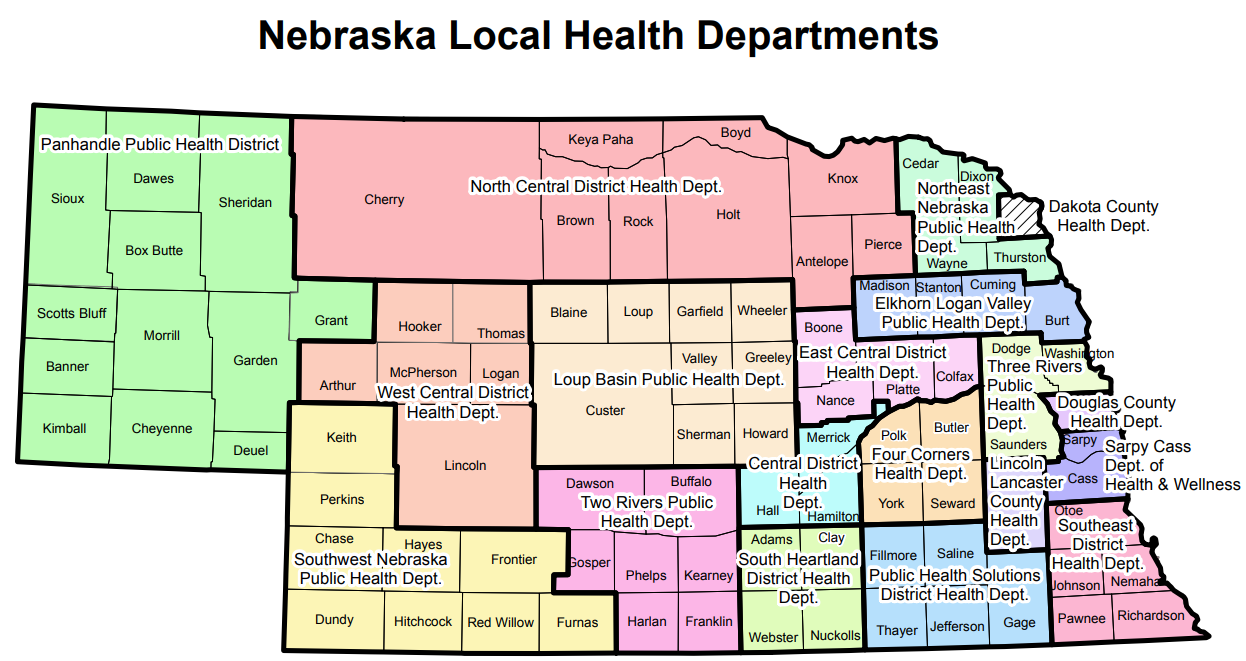 View the accessible PDF version.
View the accessible PDF version.
![]()
 Urban/Rural Categorization
Urban/Rural Categorization
Results for three urban and rural categories are presented within this dashboard when State of Nebraska is selected for the geographic region. These three categories are based on “reporting category 1" within the Disparities Demographic Data Recommendations Report, Division of Public Health, NDHHS, November 2016. The counties included within each category consist of:
Urban‐Large (7 counties)
Cass, Douglas, Lancaster, Sarpy, Saunders, Seward, Washington
Urban‐Small (15 counties)
Adams, Buffalo, Dakota, Dawson, Dixon, Dodge, Gage, Hall, Hamilton, Howard, Lincoln, Madison, Merrick, Platte, Scotts Bluff
Rural (71 counties)
Antelope, Arthur, Banner, Blaine, Boone, Box Butte, Boyd, Brown, Burt, Butler, Cedar, Chase, Cheyenne, Clay, Colfax, Cuming, Custer, Dawes, Deuel, Dundy, Fillmore, Franklin, Frontier, Furnas, Garden, Garfield, Gosper, Grant, Greeley, Harlan, Hayes, Hitchcock, Holt, Hooker, Jefferson, Johnson, Kearney, Keith, Keya Paha, Kimball, Knox, Logan, Loup, McPherson, Morrill, Nance, Nemaha, Nuckolls, Otoe, Pawnee, Perkins, Phelps, Pierce, Polk, Red Willow, Richardson, Rock, Saline, Sheridan, Sherman, Sioux, Stanton, Thayer, Thomas, Thurston, Valley, Wayne, Webster, Wheeler, York
Laboratory Surveillance
The Nebraska Laboratory Respiratory Disease Surveillance Program consists of hospital-based laboratories that submit testing data, either by weekly survey or daily electronic laboratory report (ELR). These laboratories perform rapid antigen or PCR testing for influenza, respiratory syncytial virus (RSV), and COVID-19. The Nebraska Public Health Laboratory provides further characterization of a subset of influenza isolates to determine the subtype of influenza A viruses and the lineage of influenza B viruses. Influenza A subtypes are determined by proteins, hemagglutinin (H) and neuraminidase (N), found on the outside of the virus.
For this report, influenza A subtypes are categorized into two groups, H1 and H3, as these two subtypes most commonly circulate during influenza season. Influenza B lineages are classified into one of two lineages: Yamagata and Victoria. The overall, age, patient sex, race, ethnicity, (in both 'Demographics' and 'Weekly Trends' sections) and test type data figures in the laboratory surveillance section utilizes ELR data only. The age, sex, race, and ethnicity data are obtained directly from lab reports; data missing from lab reports or specifically listed as unknown in the lab report are combined as "Unknown" in this report. If a category has no records, it is not shown in the chart/table because it is null in the database. All other data figures in this section utilize ELR data and laboratory data received via survey from Nebraska labs who do not participate in ELR. The data collected in the survey to non-ELR facilities is weekly aggregate data. Laboratory data displayed in the summary tables for influenza and RSV (positive tests and test positivity) utilize both ELR and non-ELR data described previously. For COVID-19, all laboratory data used is from ELR only.
Many respiratory disease cases are never reported. Most people with influenza, RSV, or COVID-19 do not see a doctor about their illness. Many of those who do seek care are not tested, and only a portion of test results that are obtained are reported to DHHS. DHHS receives laboratory reports from facilities participating in automated electronic laboratory reporting. We do not receive reports on all positive tests. Because some providers actively test for these respiratory diseases and others do not, relying solely on case counts could result in an incomplete assessment of respiratory disease activity in our community.
When testing for respiratory illnesses, there are two tests most used in practices. The first of the two is an antigen test, which is most common between the two. Antigen tests are inexpensive tests that generally take only 15-30 minutes to return with results. Antigen tests try to identify specific proteins on the surface of the virus. The other type of test is a polymerase chain reaction (PCR) test. This test tries to identify specific genetic material for the virus. PCR tests take longer to produce results compared to antigen tests, but it is considered the gold standard for testing because it is a lot more sensitive than the antigen test.
Note on RSV Percent Positive: An antigen test positivity of 10% and a polymerase chain reaction (PCR) test positivity of 3% are accepted threshold levels for determining when RSV activity is at an epidemic level. The healthcare community monitors these test positivity thresholds, and when they are surpassed, it indicates RSV activity is increasing throughout the population. These signals give healthcare providers more insight to know when to begin recommending monoclonal antibody treatment (i.e., Palivizumab or Nirsevimab) to infants who may be at high risk for severe illness due to RSV.
School Absenteeism Surveillance
The School Absenteeism Surveillance System captures data on the total expected enrollment at Nebraska schools, the number of total absences, and the number of absences due to specific illnesses, like influenza, RSV, and COVID-19. This surveillance system is also used to alert local health departments if absenteeism is above 10% which could indicate an outbreak situation. This system is designed to encourage communication between schools and local health departments and to promote the accessibility of Nebraska's public health system if schools need assistance, for example, with potential disease outbreaks. This data is analyzed and reported for the current surveillance week so potential outbreak situations can be identified and responded to in a timely manner.
A school closure is when an entire school is closed (all students and staff are sent home or a switch to virtual learning). A classroom closure is if the school is open for most students, but due to an outbreak cluster in a particular classroom, only the students / staff in that classroom are absent.
See more information on preventing outbreaks in schools.
Long-Term Care Facility Outbreak Surveillance
Reporting of influenza, COVID-19, and RSV outbreaks in long-term care facilities (LTCF), schools and other congregate settings is required by rules and regulations.
173 NAC 1 1-004.01B Clusters, Outbreaks, or Unusual Events, Including Possible Bioterroristic Attacks: Clusters, outbreaks, or epidemics of any health problem, infectious or other, including food poisoning, healthcare-associated outbreaks or clusters, influenza, or possible bioterroristic attack; increased disease incidence beyond expectations; unexplained deaths possibly due to unidentified infectious causes; and any unusual disease or manifestations of illness must be reported immediately.
Definition of respiratory outbreak:
A sudden increase in acute febrile respiratory illness* over the normal background rate (e.g., 2 or more cases of acute respiratory illness occurring within 72 hours of each other).
*Acute febrile respiratory illness is defined as fever > 100°F AND one or more respiratory symptoms (runny nose, sore throat, laryngitis, or cough). However, please note that elderly patients with influenza may not develop a fever. DHHS leverages ELR data to help identify respiratory disease positives in congregate settings to prompt outbreak investigations. If an outbreak is indeed identified, it gets included in our outbreak count within this dashboard for influenza, RSV, and Covid-19 respectively. Additionally, COVID-19, influenza, and RSV outbreak data is obtained from the Nebraska Infection Control Assessment and Promotion Program (ICAP) team. COVID-19 outbreak data prior to the 2024-2025 surveillance season used an outbreak definition of one or more cases to be considered an outbreak. Therefore, previous seasons reported outbreak totals may not be comparable due to the updated COVID-19 outbreak definition (listed above) effective as of the 2024-2025 season.
Read MoreShow Less
Nebraska Outpatient ILI Surveillance (ILINet)
Voluntary reporting by a statewide network of sentinel clinicians of the number of patients presenting with influenza-like illness (ILI) and the total number of patient visits by age group each week. The definition for ILI is found in previous text listed in the first “About the Data" section. This surveillance system is only used for influenza surveillance. The weekly reporting for this surveillance system ends in May, therefore no data is displayed after that point. Reporting will resume at the start of the next surveillance season around start of October.
Emergency Department and Inpatient Syndromic Surveillance
The NE Syndromic Surveillance System monitors influenza-like (ILI), COVID-like (CLI), and RSV-associated illness data received by 71/85 Nebraska emergency departments and 64/88 Nebraska inpatient facilities. Syndromic surveillance is the real-time (or near real-time) collection of patient visit data from clinics, emergency departments, hospitals, federally qualified health centers, and other healthcare facilities. Discharge diagnoses and/or chief complaint data are analyzed to identify these influenza-like, COVID-like, and RSV-associated illness visits. As mentioned previously, ILI is defined as clinically diagnosed influenza or an illness with fever of at least 100°F and cough and/or sore throat. CLI is defined as fever and cough or shortness of breath or difficulty breathing with or without the presence of a coronavirus diagnosis code. RSV-associated visit is defined as mention of 'RSV' or 'Respiratory syncytial' within chief complaint or discharge diagnosis, or identification of RSV-specific ICD-10 codes.
ILI Hospitalization Surveillance
Voluntary reporting by hospital infection preventionists of the number of hospitalizations with a diagnosis of ILI and the total number of admissions by age group each surveillance week. The definition for ILI is found in previous text listed in the first “About the Data" section. This surveillance system is only used for influenza surveillance. The weekly reporting for this surveillance system ends in May, therefore no data is displayed after that point. Reporting will resume at the start of the next surveillance season around start of October.
COVID-19 Hospitalization Surveillance
COVID-19 hospitalization data is obtained from CDC's National Healthcare Safety Network (NHSN) Hospital Respiratory Data (HRD) Reporting System, using data from both the Daily Reporting Form and Weekly Reporting Form. If a facility reported to both pathways during the same time period, the data reported to the Daily Pathway was kept. This data is aggregated with defined age groups; thus we cannot display these hospitalizations using the same age groups (0-4, 5-24, 25-49, 50-64, 65+) used elsewhere in the dashboard. This surveillance system is used for COVID-19 only. COVID-19 hospitalization data shown prior to the 2024-25 season was sourced from the federal aggregated United Hospital Data Surveillance System (UDHSS).
Mortality Surveillance
Pediatric deaths associated with influenza are required to be reported. Influenza-associated deaths in adults are not reportable. Starting in 2026, COVID-19 pediatric deaths will also be reportable in Nebraska; adult deaths will remain not reportable. RSV-associated deaths are not reportable of any age; although voluntary reporting of pediatric RSV deaths is encouraged. Voluntary reporting to public health of deaths associated with respiratory disease is encouraged to help determine the severity of the current circulating viruses. Influenza, COVID-19, and RSV deaths are obtained through the electronic death registration system (EDRS) by searching for ICD-10 codes and causes of deaths related to these diseases within death certificates. After identification of potential death in that system, our team analyzes various surveillance systems (laboratory data, medical record review) to identify a positive laboratory report. If a positive laboratory report is found on a potential death identified in the death certificate data, then it is counted in this report. This work is done in conjunction with our local health departments.